Jocelyn Étienne
Researcher of the CNRS
LIPHY—Laboratoire Interdisciplinaire de Physique
Université Grenoble Alpes
— CNRS
Course
Research
Surfing one own's wave
with Pierre Recho
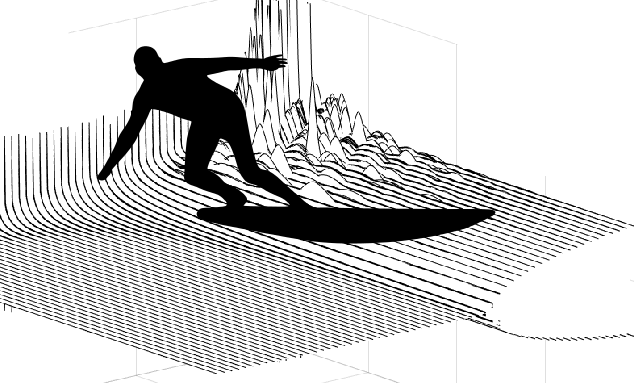
Surfers like to make waves in the media, but can only dream to make some on the surface of the ocean---and then surf them. In our paper, we show that living cells are capable of this very feat.
According to the `surfing condition' that we derive, the first requirement for this is an `active' surfboard: it needs to treadmill, that is, its surface needs to continuously move tangentially, just like a caterpillar track. One also needs that the substrate is sufficiently deformable. And finally, the friction between the surfboard and the substrate needs to decrease when the relative velocity is too large.
Cells cultured at the surface of elastic hydrogels meet all three conditions with the correct orders of magnitude of physical quantities. Their internal skeleton, namely the actin, does treadmill due to myosin molecular motors, and its adhesive interaction with the elastic substrate results in a biphasic friction law, because at high relative velocities adhesion bonds have less time to form. So they meet the surfing condition.
What's more, cells meet the surfing condition even when their treadmilling is perfectly symmetrical: that is, when their actin flows inwards (the so called retrograde flow) without any bias to define a `front' and a `back'. In that case, the trivial solution to our problem is that both the `left' and `right' side of the cell slip on the substrate and the cell remains static.
However, if the substrate is sufficiently soft, its deformations become large and this can modify the friction force... provided that the cell moves, so that these deformations are shifted in the course of time. We show that this solution exists: if the symmetric cell is moving either left or right at some adequate velocity, the deformations of the substrate move along with it as a wave, and this wave contributes to the frictional interaction... which sets the cell into motion at this precise velocity.
We show that beyond a threshold compliance of the substrate, it is these left- and right-moving solutions that are picked by the system, which thus will spontaneously break symmetry under infinitesimal perturbations and... start surfing its own wave.
Read more in our arXiv preprint: Initiation of motility on a compliant substrate
A morphoelastic model of active T1 transitions in tissue
with Alex Nestor-Bergmann,
Guy Blanchard,
Nathan Hervieux,
Bénédicte Sanson
(PDN, University of Cambridge, developmental biology) and
Alex
Fletcher (Maths, Univ Sheffield)
To go beyond what the vertex model gives access to, we have modelled cell cortices as morphoelastic Kirchhoff rods interacting via stochastic adhesion bonds with neighbouring cells. We have made the system viscoelastic by prescribing instantaneous relaxation of elastic energy. Then we have simulated active neighbour exchanges, where one junction is pre-stressed by myosin activity.
The model shows that a key parameter is the turnover time
of adhesion bonds, allowing to switch mechanical interactions from
mostly local to global, and crucially changing the dynamics.
Read more
in our
PLOS Computational Biology paper and why not run the python code yourself?.
Video below: a cell intercalation (T1) event simulated. The cortex of each cell at the vertical cell-cell junction is subject to myosin pre-stress (denoted by blue line symbol), generating a tissue-wide stress in junctions (arrows) which in turn lead to deformations, and, beyond cortex turnover time scale, flow. After the contractile junction is actively removed, a new junction extends passively.
Folding oneself into shape: Drosophila ventral furrow formation as a buckling
with Matteo Rauzi's lab (iBV Nice, CNRS, Inserm), Bénédicte Sanson's lab (PDN, University of Cambridge, developmental biology) and in LIPHY Catherine Quilliet and Philippe Marmottant
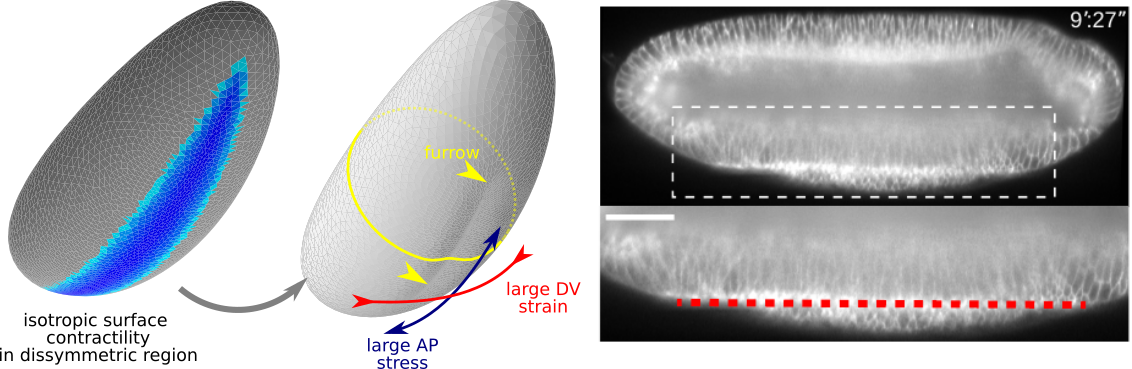
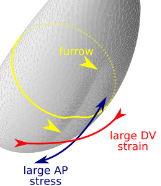
With Matteo Rauzi, we have joined two stories started separately on Drosophila ventral furrow formation. We show that an embryo-scale mechanism can fold the tissue in a straight line with a orientation prescribed by geometry
Read more in our Nature Comms paper.
Geometry can guide morphogenesis: a case of mechanical feedback
with Mahamar Dicko, Pierre Saramito (LJK Grenoble, applied mathematics), Guy Blanchard, Claire Lye and Bénédicte Sanson (PDN, University of Cambridge, developmental biology).
Would axis extension succeed if cephalic furrow had not
previously been formed? Simulations with an advanced new numerical
method, developed by Mahamar Dicko for his PhD, hint that it would
not.
Read more
in our PLOS Computational Biology paper.
Rheology of actomyosin and mechanosensing
with Atef Asnacios, Jonathan Fouchard, Démosthène Mitrossilis, Nathalie Bufi and Pauline Durand-Smet
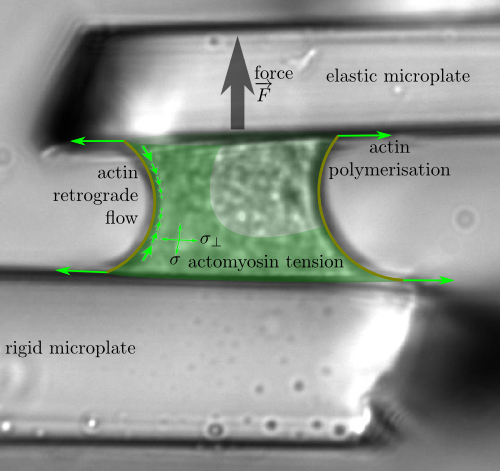 Starting from experimental observations by Atef Asnacios group (MSC, Univ. Paris
Diderot), I have
developped a model of the actomyosin dynamics which predicts quantitatively
mechanosensing experiments.
Starting from experimental observations by Atef Asnacios group (MSC, Univ. Paris
Diderot), I have
developped a model of the actomyosin dynamics which predicts quantitatively
mechanosensing experiments.
Living cells, just as muscles, exert forces on their surroundings. Although the molecules involved are the same in muscles and nonmuscle cells, their organisation is very different: crystalline sarcomeres in the former, disordered in the latter ; solid-like in the former and liquid-like in the latter. However, we show that key motor properties such as the maximum speed or the maximum load a muscle or cell can move arise from similar mechanical phenomena in both muscle and cell. Using comparisons of experiments and a rheological model's predictions, we are able to describe and quantify the energy usage of the cell when pulling on its environment, and to explain its amazing versatility and resilience.
This is now published in PNAS.
See the webpage devoted to this research, and the press release of CNRS about it (French only).
Emergent mechanical properties of the early Drosophila embryo
with Pedro Machado, Julia Duque, Alfonso Martinez-Arias, Guy Blanchard and Nicole Gorfinkiel
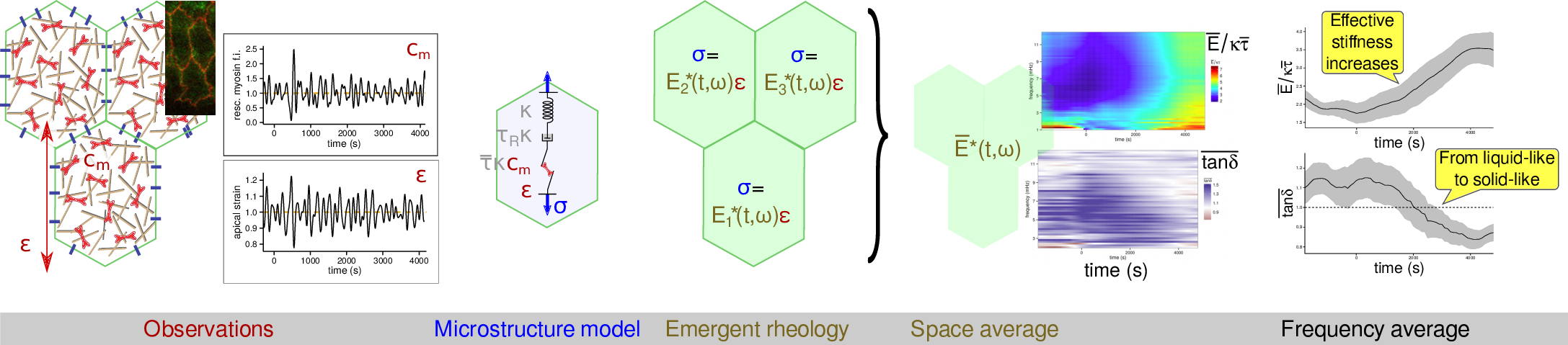
Using the microstructure-based rheology of actomyosin that I have
developped in a recent paper,
we have been able to evaluate the stress–strain ratio in
an embryonic epithelium across a few hours of development. This
ratio E* corresponds to an effective, or emergent, rheology (since
it is myosin-dependent) and we have found that it evolves in the course of
time.
Now published in BMC Biology in their “Beyond Mendel: modelling in biology” article collection.
See also our 2018 review on pulsatile actomyosin in epithelia.
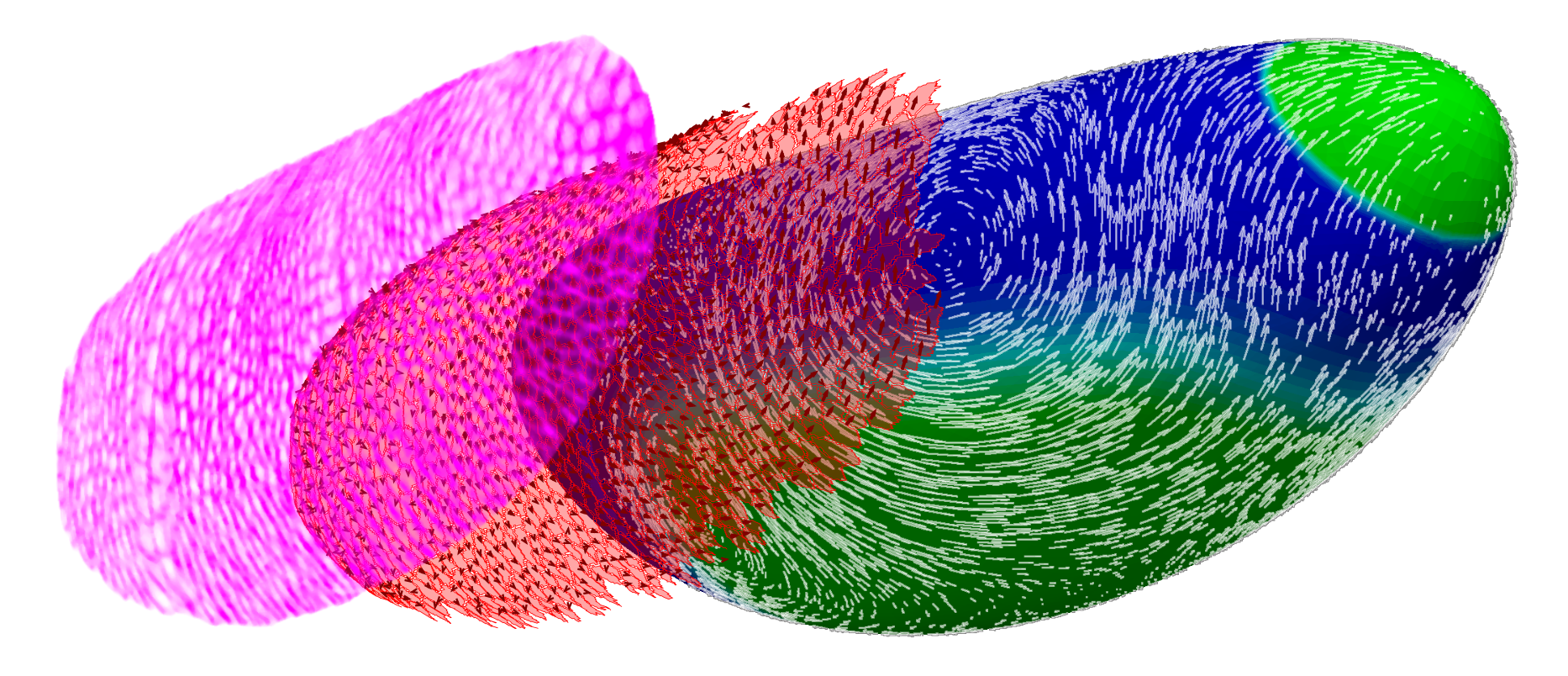
Solving flows along a curved surface
with Mahamar Dicko and Pierre Saramito
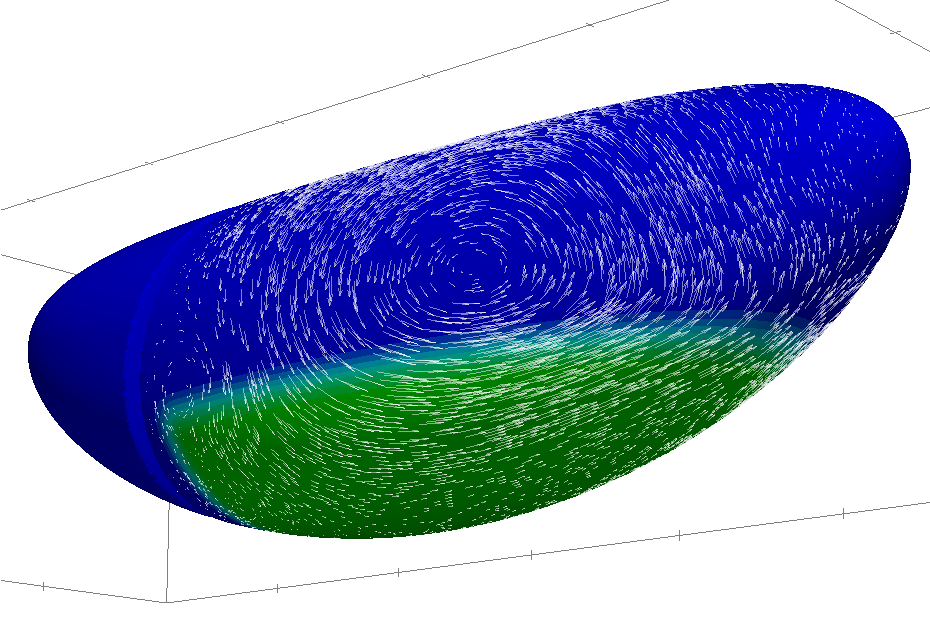 During his PhD thesis, jointly directed with Pierre in the applied math laboratory in
Grenoble, Mahamar Dicko has developed a numerical method in order to solve flows
occuring along curved deformable surfaces of the space.
During his PhD thesis, jointly directed with Pierre in the applied math laboratory in
Grenoble, Mahamar Dicko has developed a numerical method in order to solve flows
occuring along curved deformable surfaces of the space.
Preliminary work is described in Mahamar Dicko's poster (in French, poster prize at SMAI conference 2013).
This novel method is implemented as an extension of the rheolef finite element software.
Among the applications, we will focus on the mechanics of Drosophila germ band extension in collaboration with the group of Benedicte Sanson in the University of Cambridge.
The application paper on Drosophila germband extension is now in BioRxiv.
Dynamics of initial cell spreading
With Alain Duperray
In [Biophys J 2011], we show that the initial spreading of cells is governed by the rate at which gains of adhesion energy can deform the actin cortex, e.g. by breakage of crosslinks.
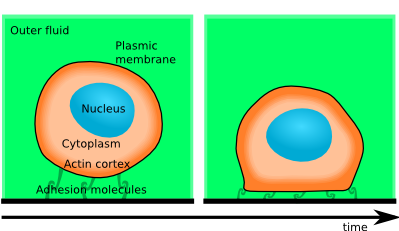
Just after contact with a substrate, cells are found to spread at a rate described by a sequence of power-laws, which is independent of the spatial scale (that is, it scales with the cell size). Thus the observed dynamics can only be explained by cell-scale phenomena, which are of mechanical nature. This means that spreading rate can be used to test apparent rheological properties of the live cell at a time-scale of 10 to 100 seconds, which is the relevant time scale of a large number of intriguing active dynamics of cells.
By testing a number of simplified models, I have demonstrated that the leading order balance that governs this dynamics is between the adhesion force and the dissipation incurred by the actin cortex during the shape changes required by cell spreading. This observed dissipation is the trace of several dissipative phenomena, which include the energetic cost of cross-linking and uncross-linking the actin bundles and viscous drag.
Here is a poster presenting this for the conference Cell Mechanics in Amsterdam (October 2011).
Scientific community
Mastodon
I believe social media can be useful to science, but need to be free from the influence of economic interests. Mastodon offers both an adequate environment and the necessary guarantees of this, and hosts now a bustling scientific community. I have two accounts there with a relevance to my scientific activity: one on Mathstodon for topics strictly related with my research (see left margin for latest posts) and another on Scicomm for posts related to the scientific community as a whole (samples below).